
INNOVATION THROUGH TECHNOLOGY
Neurelis was founded in 2007 with a vow to provide progressive solutions in therapeutic care to address unmet needs. Our technologies help to accelerate product development and enhance drug performance. By optimizing the development pathway and improving product performance, we empower people and their healthcare providers with a broader array of treatment options.
Neurelis Technology Portfolio

Intravail®
SEE HOW INTRAVAIL® OPTIMIZES ABSORPTION
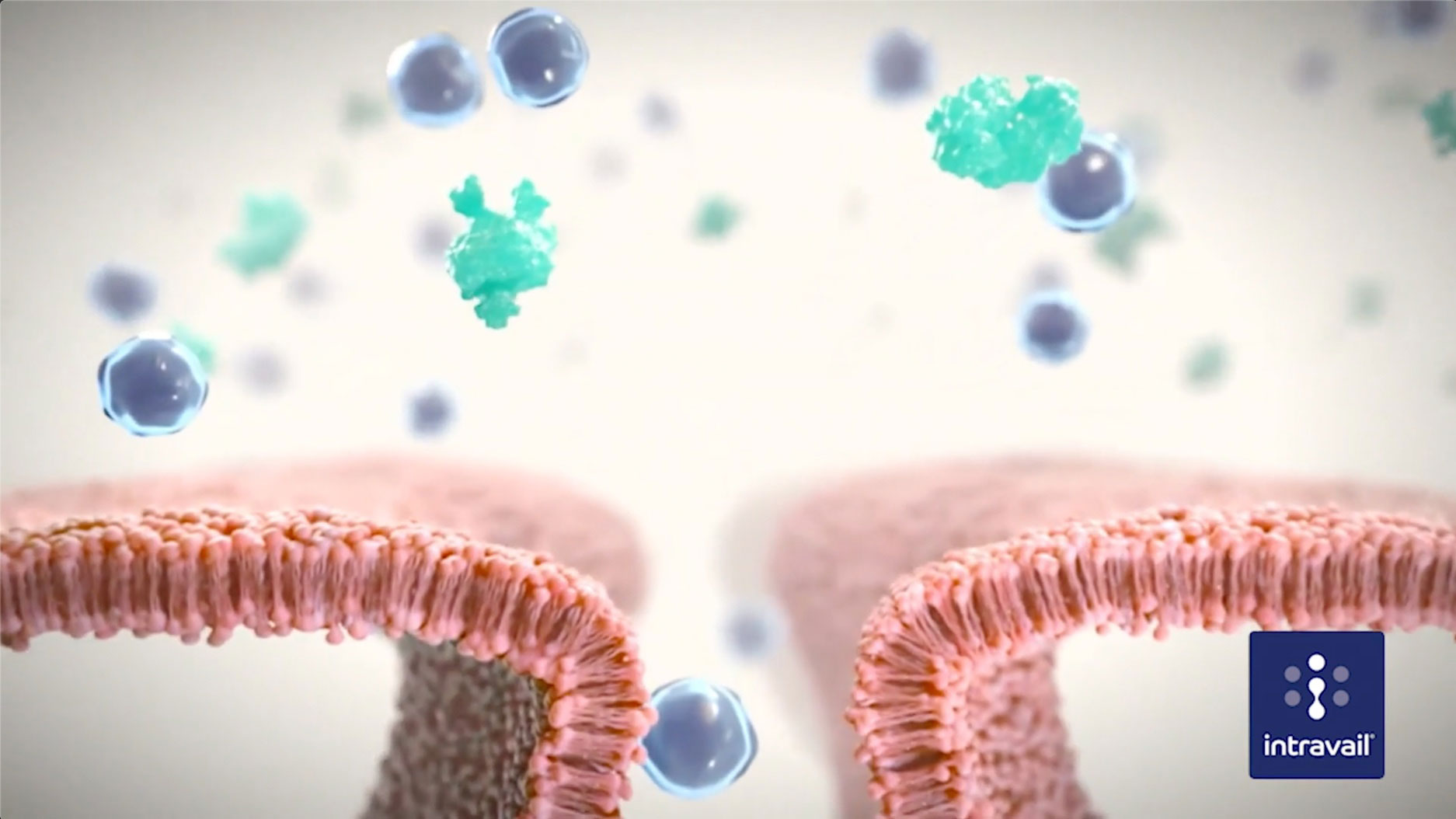
THE ADVANTAGES OF INTRAVAIL®:
- Increased bioavailability
Addressing sub-optimal drug absorption - Application to wide range of molecules
Small molecules, therapeutic proteins, peptides, non-peptide macromolecules (up to ~ 30KDa)
- Safety
Odorless, tasteless, non-toxic, non-mutagenic, and non-irritating - Solubility in water and oils
Compatible with routine liquid formulation and dispensing processes for ease of scale-up and production

Protek®
ProTek protein technology aids in stabilization that prevents the clustering of proteins and peptides, thus reducing immunogenicity. ProTek allows the creation of easily manufacturable, homogeneous, stable, aqueous or lyophilized dosage forms for therapeutics that maintain the structural integrity and physiological activity of many protein and peptide drugs. Formulations are applicable to injectable, intranasal, and other dosage forms.

Hydrogel™
Hydrogel is an absorption-enhancing water-based gel used for transdermal and transmucosal drug delivery applications. Hydrogel serves simultaneously as a delivery vehicle and absorption enhancer. In dermal applications, Hydrogel leaves the skin feeling soft without residue. The thixotropic nature of Hydrogel prevents dripping and running when dispensing, which allows the gel to self-stabilize as soon as it is applied to the affected area.
Supporting Publications
Download the supporting publications below for more information on the Neurelis Technology Portfolio
Bioequivalence of biotherapeutics – looking beyond bioavailability and pharmacodynamics DOWNLOAD PDF
Circumventing polysorbate induced unwanted immunogenicity and anaphylaxis DOWNLOAD PDF
Reducing or eliminating polysorbate induced anaphylaxis and unwanted immunogenicity in biotherapeutics DOWNLOAD PDF
Polysorbates, biotherapeutics, and anaphylaxis DOWNLOAD PDF
Alkyl mono- and diglucosides: highly effective, nonionic surfactant replacements for polysorbates in biotherapeutics—a review DOWNLOAD PDF
Absorption enhancing excipients in nasal drug delivery DOWNLOAD PDF
Biosimilars, oxidative damage, and unwanted immunogenicity DOWLOAD PDF
Highly bioavailable nasal calcitonin – potential for expanded use in analgesia DOWNLOAD PDF
Novel excipients prevent aggregation in manufacturing and formulation of protein and peptide therapeutics DOWNLOAD PDF
